Purpose Statement
The Lansing Community College Institutional Review Board (IRB) exists to protect the welfare, rights, and safety, of human subjects involved in research at LCC. IRB and HS-IRB (Human Subjects Institutional Review Board) are used interchangeably throughout this site.
The College's IRB is responsible for review and approval of all behavioral or biomedical research activities involving human subjects at LCC, for LCC, or with LCC students and/or employees, with the core purpose of ensuring the research meets ethical standards for human subjects involved in such research.
The college's IRB approval is required prior to conducting research involving human subjects at LCC, for LCC, and/or with LCC students and/or employees.
Please note that additional approval may be required by the division or department head prior to beginning your research activities.
Form and Template Links
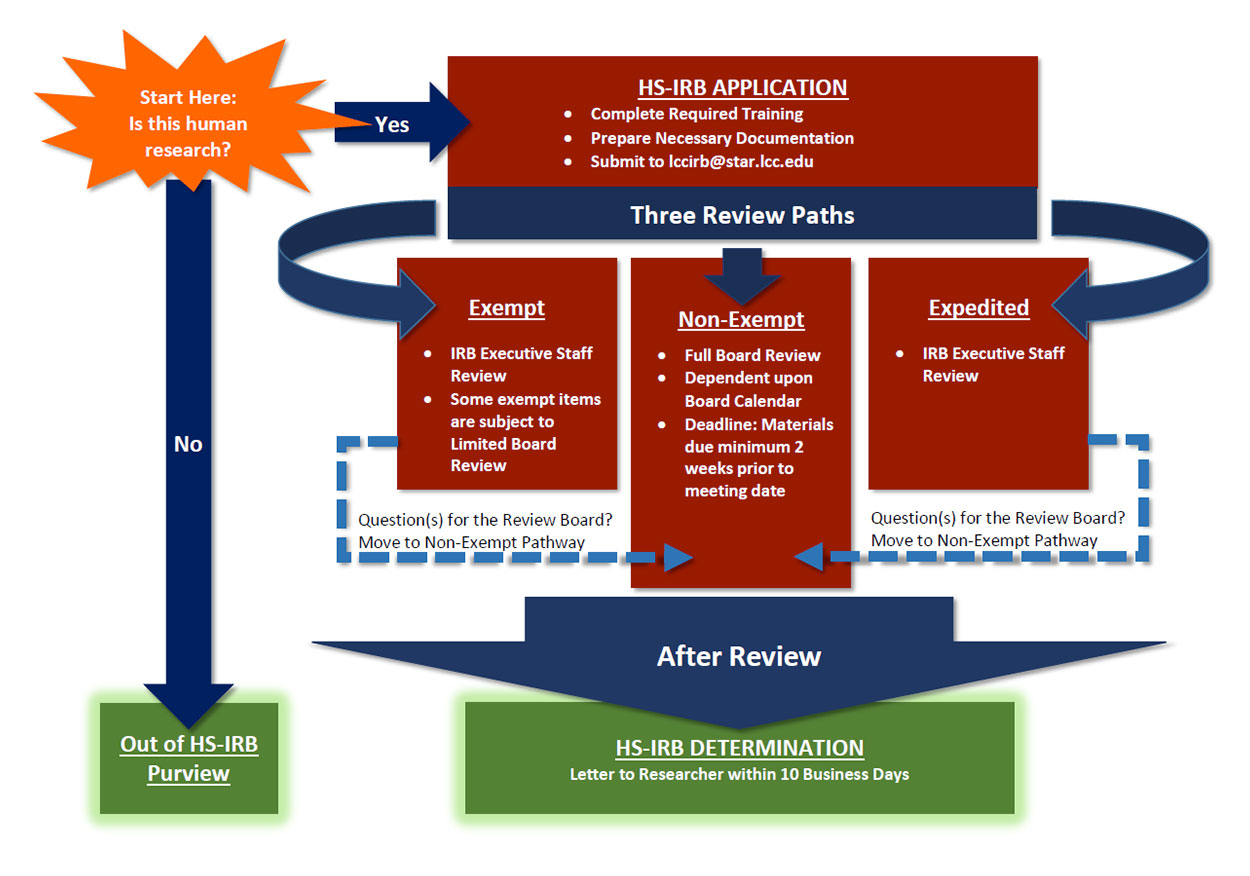
IRB Research Application
The Primary Investigator (PI) must submit a new IRB Application, and associated attachments, for review by the IRB. This is for any research involving LCC employees or students – whether as investigators or research participants.
You can also complete and submit this form to request an IRB review and determination of whether the research falls under HS-IRB purview.
Word File IRB research applicationInformed Consent Checklist
This resource serves as a guide for researchers when drafting informed consent forms, outlining the key elements required for participant consent.
Excel Spreadsheet File Informed Consent checklistContinuing Review Form
This form is only used for existing approved HS-IRB research projects. It must be completed and submitted at least six weeks before the current HS-IRB approval expires.
Word File Continuing Review formSurvey Confidentiality Statement
This statement was created by the HS-IRB per request, and can be used on LCC distributed surveys. It is generally worded to cover many conditions. Please feel free to copy and paste the information in this document into your surveys.
Word File Survey Confidentiality statementIRB and Human Subjects Research
Not all activities will need IRB approval. The IRB follows US Federal Department of Health and Human Services (HHS) regulations as determined by the Office for Human Research Protections (OHRP) and laid out in 45 CFR (Code of Federal Regulations) 46. Subpart A within these regulations is known as “The Common Rule."
Research, in IRB terms, involves human subjects and is defined by the Common Rule as, “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.”
The following lists contain examples to help clarify the research that needs to involve our LCC Human Subjects Institutional Research Board (HS-IRB) and research that does not need IRB approval. However, if you have any questions do not hesitate to contact the HS-IRB at lccirb@star.lcc.edu . We’re here to help!”
Activity that DOES require IRB approval
IRB approval is required anytime human subjects are involved in:
- Research that is a systematic investigation (including research development, testing, and evaluation), designed to develop or contribute to generalizable knowledge to a larger group.
- Research conducted, supported, or funded by federal, state, or local agencies or otherwise supported by an agency not affiliated with Lansing Community College.
- Research in which the intent is to disseminate the finding(s) to a scientific audience (national, regional, state, local, or college meetings, conferences or presentations) or submit the results of the investigation to a scientific publication, electronic journal, or internet posting.
- Research that involves private information, or biological or pathological specimens that can be linked to an individual.
- Research involved with capstone/thesis research projects or independent class projects.
Activity that MAY NOT require IRB approval
IRB approval may not be required in some cases; however, if you have any questions or are unsure about your situation, please reach out to the IRB for clarification.
- Course-related activities or assignments for educational teaching purposes that are part of the course and the information collected is not disseminated for use outside the classroom (e.g. labs, projects, class exercises). The assignment can include a presentation of data collected to the class, but the data must be destroyed at the end of the project.
- Data collection for internal department or other college administrative purposes (e.g. course evaluations, employee and/or student satisfaction surveys).
- Surveys issued or conducted by college employees for the purpose of improving services
and programs of the college, or for developing new services or programs for students,
employees, and/or alumni.
- Note: If you are running a survey that will be distributed to more than 50 students and/or employees, or across multiple departments at LCC, please be aware that you must adhere to LCC Standard Operating Procedure (SOP) ‘Surveys of Large Scale’.
- Informational gathering meetings, interviews, or surveys that focus on college processes, services, or policies (i.e. quality improvement and quality assurance surveys) unless clear intent is for generalizable knowledge.
- College evaluations that are conducted under independent contract by an external agency for internal college purposes only (e.g. employee studies, customer satisfaction surveys, cost-benefit analysis, program enrollment, constituent demographics, and outcome analysis).
- Items falling under Federal regulations classified as “not research”
- Oral history, journalism, biography, literary criticism, legal research, and historical scholarship focused on specific individuals.
Research Decision Trees
The Office for Human Research Protections (OHRP) provides decision charts to help determine if a project falls under IRB review or qualifies for exemption. Use the link below to access these charts in HTML format or download PDFs.
While the terminology can be complex, the general lists above offer guidance. LCC’s HS-IRB often uses these decision trees as a review rubric. If you've completed basic primary researcher training, the terms should be familiar.
OHRP DECISION TREESMinimal Training Requirement for Primary Researchers
The HS-IRB requires primary researchers to complete minimal training before project approval or exemption. Proof of completion must be submitted via a Certificate of Completion from one of the training options below. The certificate must be dated within three years of the proposal submission. Submitted proof can be a PDF, screen shot, or scanned printed copy.
Minimal Training Options (Choose One)
- Training provided by the researcher’s home institution.
- Free Human Research Protection Foundational training - U.S. Department of Health and Human Services, Office for Human Research Protections
(OHRP).
- Lesson: When HHS Regulations Apply (Lesson 1) (Approx. 35 minutes)
- Steps:
- Access Link: OHRP Human Research Protection Foundational Training
- Select Lesson 1
- Complete the lesson and submit proof of the certificate.
- CITI Training for investigators (Free for LCC Employees and Students).
- Group 3: Students (General overview, Approx. 60 minutes)
or - Group 2: Social, Behavioral, Educational Researchers (In-depth training, Approx. 10 hours)
- How to Access CITI Training:
- Visit the CITI Program Website
- Click Register, select Lansing Community College as the organization
- After registering, click View Courses
- Scroll to “Learner Tools for Lansing Community College,” then Click “Add a Course”
- Required trainings are listed under Question 1
- Select a course, scroll down, and click Submit
- Complete the course and submit proof of the certificate.
- Group 3: Students (General overview, Approx. 60 minutes)
For questions, contact HS-IRB at lccirb@star.lcc.edu.
LCC HS-IRB Calendar of Meetings
All meetings of the HS-IRB are open to the public and will be held in the Administration Building (ADM), room 105, from 9:10am - 10:30am unless otherwise indicated. A copy of the upcoming meeting materials can be obtained from the Center for Data Science office, by calling 517-483-1123 or emailing lccirb@lcc.edu. Information pertaining to meetings held before the current academic year can also be obtained by contacting the Center for Data Science.
Academic Year 2025 - 2026
September 12, 2025
Agenda | Minutes | NOTICE
October 21, 2025
Agenda | Minutes | NOTICE
November 7, 2025 - Canceled
December 5, 2025 - Canceled
January 16, 2026 - Canceled
February 13, 2026
Agenda | Minutes | NOTICE
March 13, 2026 - Canceled
April 10, 2026
Agenda | Minutes | NOTICE
May 8, 2026
Agenda | Minutes | NOTICE
HS-IRB Information
Chairperson
Melinda Wilson, PhD
Email: lccirb@lcc.edu
Vice-Chairperson
Heather Bunce, EdD
Email: lccirb@lcc.edu
Administrator
Matthew Fall, PhD
Email: lccirb@lcc.edu
HS-IRB Members
- Patti Ayers, BA, Student Ombuds
- Robert Halgren, PhD
- Kali Majumdar, PhD
- Wyl McCully, PhD
- Margherita Clark, MSN, RN, GNP-BC, HS-IRB Community Member